HigherPurity™ Viral DNA/RNA Kit
For a Highly Efficient, Safe & Rapid simultaneous Purification of Viral DNA and RNA from cell–free samples
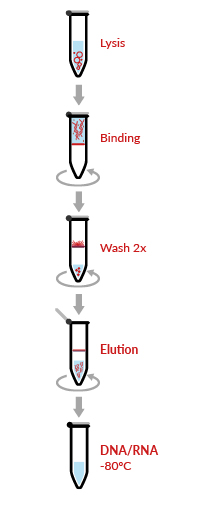
HigherPurity™ Viral DNA/RNA Extraction Kit offers an highly efficient, fast and simultaneous purification of viral DNA and RNA from cell–free samples such as serum, plasma and cerebrospinal fluid. The kit uses HigherPurity™ breakthrough technology based in nucleic acid ability to bind silica in the presence of high concentrations of chaotropic salts. The viral RNA/DNA molecules bind to the silica-based media and impurities such as proteins and nucleases are removed by thorough washing with Wash Buffer. The RNA/DNA is then eluted in sterile, RNase free water.
Price: 349.89 € – 876.29 €